| Active Ingredient | PAZOPANIB HYDROCHLORIDE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VOTRIENT | (NDA) 022465 | NOVARTIS PHARMS CORP | TABLET;ORAL | EQ 200MG BASE | EQ 200MG BASE (RS) | October 19, 2009 | - | April 26, 2019 | 1 New molecular entity (NME) | S Standard review drug | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
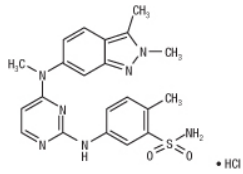 |
| Chemical Name | 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2pyrimidinyl]amino]-2-methylbenzenesulfonamide monohydrochloride |
| CAS No | 444731-52-6 |
| Molecular Formula | C21H23N7O2S•HCl |
| Molecular Weight | 473.99 |
| Appearance | a white to slightly yellow crystalline solid |
| Solubility | Pazopanib is very slightly soluble in aqueous solutions pH 1, being practically insoluble above pH 4. In order to improve its solubility, the active substance is micronized. |
| Water Solubility | 0.0433 mg/mL (Predicted) |
| Polymorphism | The crystal structure has been determined by single crystal X-ray crystallography. The solid state form selected is Form 1. It has been shown that the proposed manufacturing process consistently produces Form 1 and that no conversion occurs during stability. |
| pKa (Strongest Acidic) | 10.41 (Predicted) |
| pKa (Strongest Basic) | 5.07 (Predicted) |
| Log P | 3.59 (Predicted) |
| Identification | IR, chloride counter ion |
| Degradation | API shows no significant degradation in any stress condition |
| Hygroscopic | Non hygroscopic |
| Photostability study | Photo stable |
| Melting Point | - |
| BCS Class | II |
| Manufacture of API | Pazopanib is synthesized in a four step process using acommercially available starting material. The choice of the starting material as well as the route of synthesis has been appropriately justified. The applicant has used risk assessment and Design of Experiments to identify the critical process parameters that affect the critical quality attributes of the active substance as well as to establish the operating ranges that will ensure the desired product quality. |
| Parameters | Details |
|---|---|
| Indications and Usage | VOTRIENT is indicated for the treatment of patients with advanced renal cell carcinoma (RCC). VOTRIENT is indicated for the treatment of patients with advanced soft tissue sarcoma (STS) who have received prior chemotherapy. Limitation of Use: The efficacy of VOTRIENT for the treatment of patients with adipocytic STS or gastrointestinal stromal tumors has not been demonstrated. |
| Dosage and Administration |
The recommended starting dose of VOTRIENT is 800 mg orally once daily without food (at least 1 hour before or 2 hours after a meal). The dose of VOTRIENT should not exceed 800 mg. Do not crush tablets due to the potential for increased rate of absorption which may affect systemic exposure. If a dose is missed, it should not be taken if it is less than 12 hours until the next dose. |
| Mechanism of action | Pazopanib is a multi-tyrosine kinase inhibitor of vascular endothelial growth factor receptor (VEGFR) 1, VEGFR-2, VEGFR-3, platelet-derived growth factor receptor (PDGFR)-α and -β, fibroblast growth factor receptor (FGFR)-1 and -3, cytokine receptor (Kit), interleukin-2 receptor-inducible T-cell kinase (Itk), leukocyte-specific protein tyrosine kinase (Lck), and transmembrane glycoprotein receptor tyrosine kinase (cFms). In vitro, pazopanib inhibited ligand-induced autophosphorylation of VEGFR-2, Kit, and PDGFR-β receptors. In vivo, pazopanib inhibited VEGF-induced VEGFR-2 phosphorylation in mouse lungs, angiogenesis in a mouse model, and the growth of some human tumor xenografts in mice. |
| Absorption |
Pazopanib is absorbed orally with median time to achieve peak concentrations of 2 to 4 hours after the dose. Daily dosing at 800 mg results in geometric mean AUC and Cma x of 1,037 mcg•h/mL and 58.1 mcg/mL (equivalent to 132 µM), respectively. There was no consistent increase in AUC or Cma x at pazopanib doses above 800 mg. Administration of a single pazopanib 400-mg crushed tablet increased AUC(0-72) by 46% and Cma x by approximately 2 fold and decreased Tma x by approximately 2 hours compared with administration of the whole tablet. These results indicate that the bioavailability and the rate of pazopanib oral absorption are increased after administration of the crushed tablet relative to administration of the whole tablet. Therefore, due to this potential for increased exposure, tablets of VOTRIENT should not be crushed. |
| Food Effect | Systemic exposure to pazopanib is increased when administered with food. Administration of pazopanib with a high-fat or low-fat meal results in an approximately 2-fold increase in AUC and Cmax. Therefore, pazopanib should be administered at least 1 hour before or 2 hours after a meal. |
| Distribution | Binding of pazopanib to human plasma protein in vivo was greater than 99% with no concentration dependence over the range of 10 to 100 mcg/mL. In vitro studies suggest that pazopanib is a substrate for P-gp and BCRP. |
| Metabolism | In vitro studies demonstrated that pazopanib is metabolized by CYP3A4 with a minor contribution from CYP1A2 and CYP2C8. |
| Elimination | Pazopanib has a mean half-life of 30.9 hours after administration of the recommended dose of 800 mg. Elimination is primarily via feces with renal elimination accounting for <4% of the administered dose. |
| Peak plasma time (Tmax) | 2 to 4 hours |
| Half life | 30.9 hours |
| Bioavailability | - |
| Age, gender | - |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| 26207 | A | II | October 7, 2012 | HETERO LABS LTD |
| 27422 | A | II | August 31, 2013 | MSN LABORATORIES PRIVATE LTD |
| 31297 | A | II | January 3, 2017 | FORMOSA LABORATORIES INC |
| Parameters | Details |
|---|---|
| Strength | 216.7 mg of pazopanib hydrochloride equivalent to 200 mg of pazopanib |
| Excipients used |
Magnesium stearate (2.1MG), microcrystalline cellulose (64MG), povidone K30 (16MG), sodium starch glycolate (21.2MG) |
| Composition of coating material | Gray film-coat (9.6MG): Hypromellose, iron oxide black (0.13MG), macrogol/polyethylene glycol 400 (PEG 400), polysorbate 80, titanium dioxide |
| Composition of caspule shell | - |
| Pharmaceutical Development |
The active substance can be classified as a Class II active substance (high permeability, low solubility) according to the Biopharmaceutics Classification System (BCS). This implies that particle size is expected to impact dissolution and thus bioavailability. Therefore the active substance is micronized in order to improve its solubility and to provide a more consistent particle size input to the granulation process. In addition, considerable attention was paid to the development of a discriminating dissolution method. Based on risk assessment, the following attributes of the active substance were identified as critical for the finished product quality; solubility, particle size distribution, identity, form, and impurities. Multivariate analysis (MVA) was conducted to evaluate the variability in the input active substance used for the manufacture of clinical trial batches. In all cases the manufacturing process could manage the variability in the active substance attributes providing fi nished product with consistent performance. A standard wet granulation method was used for the manufacture of the finished product. Extensive development studies were performed to establish the critical process parameters that affect finished product quality. |
| Manufacture of the product | The manufacturing process is a standard wet granulation process and consists of the following steps: granulation; milling; drying; blending and compression. The tablets are then film-coated. |
| Tablet / Capsule Image |
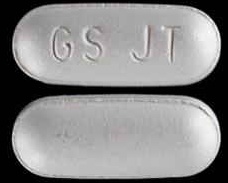
|
| Appearance | modified capsule-shaped, gray, film-coated with GS JT debossed on one side |
| Imprint code / Engraving / Debossment | GS JT debossed on one side and plain at other side |
| Score | no score |
| Color | GRAY |
| Shape | FREEFORM (capsule-shaped) |
| Dimension | 14mm |
| Mfg by | GlaxoSmithKline (EU) |
| Mfg for | - |
| Marketed by | GlaxoSmithKline (US), Novartis (EU) |
| Distributed by | - |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N022465 | 1 | 7105530 | October 19, 2023 | Y | Y | - | - | Download |
| N022465 | 1 | 7262203 | December 19, 2021 | Y | Y | - | - | Download |
| N022465 | 1 | 8114885 | December 19, 2021 | Y | Y | - | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 75 | 50 mM Sodium Acetate buffer, pH 4.5, containing 0.75% SDS | 900 | 10, 15, 30, 45 and 60 | August 5, 2010 |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | Download |
| FDA BE Recommendation | Download |
| European Public Assessment Report | Download |
| Territory | Brand name / Generic company name | Link |
|---|---|---|
| EU | VOTRIENT | Download |
| UK | VOTRIENT | Download |
| US | VOTRIENT | Download |
| VOTRIENT tablet 200MG as well as 400MG both strength are approved in EU and UK. |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
